Formation of Orotic Acid
Formation of Orotic Acid
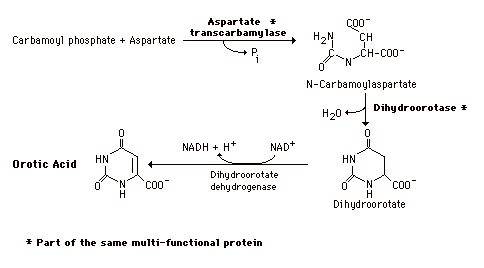
During the formation of Orotic Acid Carbamoyl phosphate condenses with aspartate in the presence of aspartate transcarbamylase to yield N-carbamylaspartate which is then converted to dihydroorotate. In man, CPSII, asp-transcarbamylase, and dihydroorotase activities are part of a multifunctional protein. Oxidation of the ring by a complex, poorly understood enzyme produces the free pyrimidine, orotic acid. This enzyme is located on the outer face of the inner mitochondrial membrane, in contrast to the other enzymes which are cytosolic. Note in the Formation of the Orotic acid the contrast with purine synthesis in which a nucleotide is formed first while pyrimidines are first synthesized as the free base.
Graphic - formation of Oric Acid
Formation of the Nucleotides
Formation of Orotic Acid
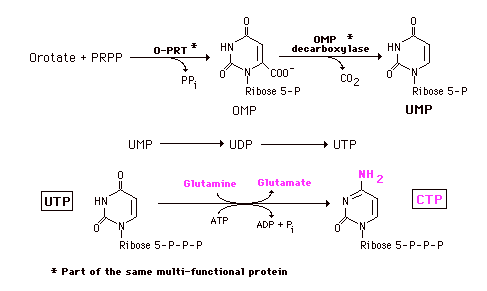
Orotic acid is converted to its nucleotide with PRPP. OMP is then converted sequentially - not in a branched pathway - to the other pyrimidine nucleotides. Decarboxylation of OMP gives UMP. O-PRT and OMP decarboxylase are also a multifunctional protein. After conversion of UMP to the triphosphate, the amide of glutamine is added, at the expense of ATP, to yield CTP.
Control
The control of pyrimidine nucleotide synthesis in man is exerted primarily at the level of cytoplasmic CPS II. UTP inhibits the enzyme, competitively with ATP. PRPP activates it. Other secondary sites of control also exist (e.g. OMP decarboxylase is inhibited by UMP and CMP). These are probably not very important under normal circumstances. In bacteria, aspartate transcarbamylase is the control enzyme. There is only one carbamoyl phosphate synthetase in bacteria since they do not have mitochondria. Carbamoyl phosphate, thus, participates in a branched pathway in these organisms that leads to either pyrimidine nucleotides or arginine.
Formation of Orotic Acid
Interconversion of Nucleotides
The monophosphates are the forms synthesized de novo although the triphosphates are the most commonly used forms. But, of course, the three forms are in equilibrium. There are several enzymes classified as nucleoside monophosphate kinases which catalyze the general reaction:(= represents a reversible reaction) Base-monophosphate + ATP = Base-diphosphate + ADP e.g. Adenylate kinase: AMP + ATP = 2 ADP There is a different enzyme for GMP, one for pyrimidines and also enzymes that recognize the deoxy forms. Similarly, the diphosphates are converted to the triphosphates by nucleoside diphosphate kinase: BDP + ATP = BTP + ADP There may be only one nucleoside diphosphate kinase with broad specificity. One can legitimately speak of a pool of nucleotides in equilibrium with each other.
Salvage of Bases
Salvaging of purine and pyrimidine bases is an exceedingly important process for most tissues. There are two distinct pathways possible for salvaging the bases.
Salvaging Purines The more important of the pathways for salvaging purines uses enzymes called phosphoribosyltransferases (PRT): PRTs catalyze the addition of ribose 5-phosphate to the base from PRPP to yield a nucleotide.: Base + PRPP = Base-ribose-phosphate (BMP) + PPi We gave already seen one example of this type of enzyme as a normal part of de novo synthesis of the pyrimidine nucleotides, - O-PRT. As a salvage process though, we are dealing with purines. There are two enzymes, A-PRT and HG-PRT. A-PRT is not very important because we generate very little adenine. (Remember that the catabolism of adenine nucleotides and nucleosides is through inosine). HG-PRT, though, is exceptionally important and it is inhibited by both IMP and GMP. This enzyme salvages guanine directly and adenine indirectly. Remember that AMP is generated primarily from IMP, not from free adenine.
Lesch-Nyhan Syndrome HG-PRT is deficient in the disease called Lesch-Nyhan Syndrome, a severe neurological disorder whose most blatant clinical manifestation is an uncontrollable self-mutilation. Lesch-Nyhan patients have very high blood uric acid levels because of an essentially uncontrolled de novo synthesis. (It can be as much as 20 times the normal rate). There is a significant increase in PRPP levels in various cells and an inability to maintain levels of IMP and GMP via salvage pathways. Both of these factors could lead to an increase in the activity of the amidotransferase.
Salvaging Pyrimidines A second type of salvage pathway involves two steps and is the major pathway for the pyrimidines, uracil and thymine. Base + Ribose 1-phosphate = Nucleoside + Pi (nucleoside phosphorylase) Nucleoside + ATP - Nucleotide + ADP (nucleoside kinase - irreversible) There is a uridine phosphorylase and kinase and a deoxythymidine phosphorylase and a thymidine kinase which can salvage some thymine in the presence of dR 1-P.
Formation of Orotic Acid
Formation of Deoxyribonucleotides
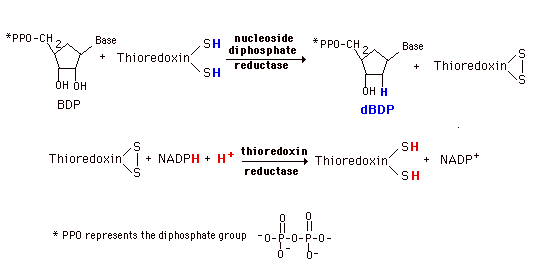
De novo synthesis and most of the salvage pathways involve the ribonucleotides. (Exception is the small amount of salvage of thymine indicated above.) Deoxyribonucleotides for DNA synthesis are formed from the ribonucleotide diphosphates (in mammals and E. coli). A base diphosphate (BDP) is reduced at the 2' position of the ribose portion using the protein, thioredoxin and the enzyme nucleoside diphosphate reductase. Thioredoxin has two sulfhydryl groups which are oxidized to a disulfide bond during the process. In order to restore the thioredoxin to its reduced for so that it can be reused, thioredoxin reductase and NADPH are required.

This system is very tightly controlled by a variety of allosteric effectors. dATP is a general inhibitor for all substrates and ATP an activator. Each substrate then has a specific positive effector (a BTP or dBTP). The result is a maintenance of an appropriate balance of the deoxynucleotides for DNA synthesis.
Formation of Orotic Acid
Synthesis of dTMP
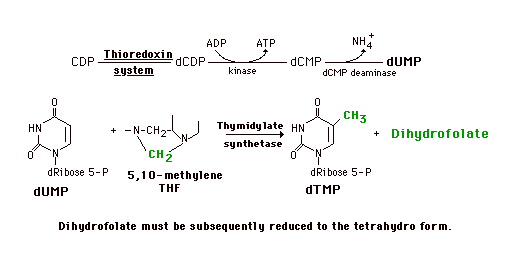
DNA synthesis also requires dTMP (dTTP). This is not synthesized in the de novo pathway and salvage is not adequate to maintain the necessary amount. dTMP is generated from dUMP using the folate-dependent one-carbon pool. Since the nucleoside diphosphate reductase is not very active toward UDP, CDP is reduced to dCDP which is converted to dCMP. This is then deaminated to form dUMP. In the presence of 5,10-Methylene tetrahydrofolate and the enzyme thymidylate synthetase, the carbon group is both transferred to the pyrimidine ring and further reduced to a methyl group. The other product is dihydrofolate which is subsequently reduced to the tetrahydrofolate by dihydrofolate reductase.
Chemotherapeutic Agents
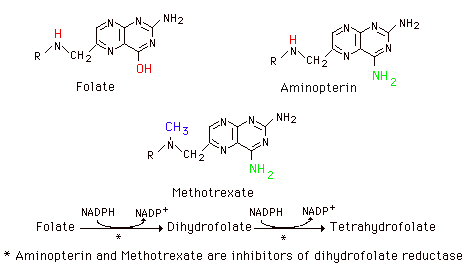
Thymidylate synthetase is particularly sensitive to availability of the folate one-carbon pool. Some of the cancer chemotherapeutic agents interfere with this process as well as with the steps in purine nucleotide synthesis involving the pool. Cancer chemotherapeutic agents like methotrexate (4-amino, 10-methyl folic acid) and aminopterin (4-amino, folic acid) are structural analogs of folic acid and inhibit dihydrofolate reductase. This interferes with maintenance of the folate pool and thus of de novo synthesis of purine nucleotides and of dTMP synthesis. Such agents are highly toxic and administered under careful control.
Formation of Orotic Acid
Reprint Permission Given to Gout-Aware All Articles written & supplied by
Dr Carol .N. Angsatdt Ph.D Department of Biomedical Sciences Allegheny University of Health Sciences

Recent Articles
-
Tony
May 08, 24 08:57 PM
Hi Peter, I am a physician, had my first Gout episode last week. I was surfing through the web and happened to come across your site. I am impressed by -
What is it about Cherries?
Aug 25, 21 03:59 AM
Allopurinol worked for me, but at the expense of adversely affecting my mood.I like cherries, but getting them every day of the year is a problem.I wondered -
ACV made my gout worse...
Apr 22, 20 07:19 AM
During my first gout attack, I was in so much pain that I desperately scoured the internet for alternative cures as the colchicine that I was prescribed


